My techniques for the indoor cultivation and propagation of Heliamphora.
Testing for pollen viability (under construction)
Early on one of the hurdles in having a good understanding of Heliamphora pollination was understanding what was wrong when things didn't work. It was obvious that I needed techniques to do a failure root cause analysis.
This primarily consisted of understanding the viability of pollen. I dug around and tried several standard methods of growing pollen tubes using sucrose based formulations. However, none of these standard formulations i tried worked for Heliamphora. I finally came across a technique where pollen tubes were grown on the epidermis of the genus Allium.
I removed the epidermis, placed it on a microscope slide, sprinkled some pollen on it and placed the slide inside a DIY incubator. Two hours later I pulled the slide from the incubator and had a look.
I couldn't believe what i was seeing. After so many failures using "textbook" Sucrose formulations, I had finally succeeded. I could now accurately test Heliamphora pollen, and I was the first person to do so as far as I knew.
Here are some of my early pictures of Heliamphora pollen tube growth.
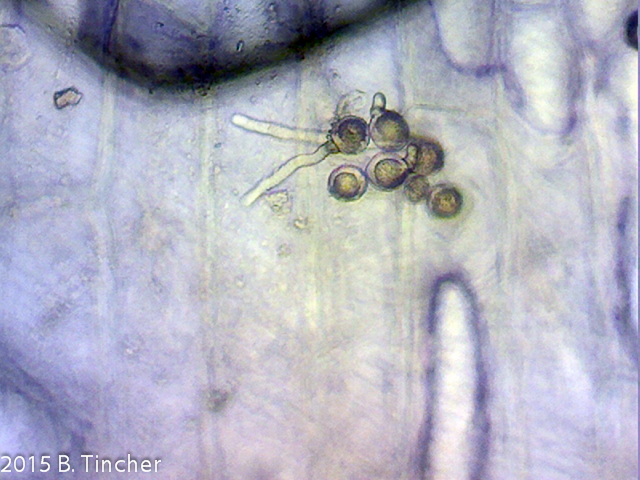
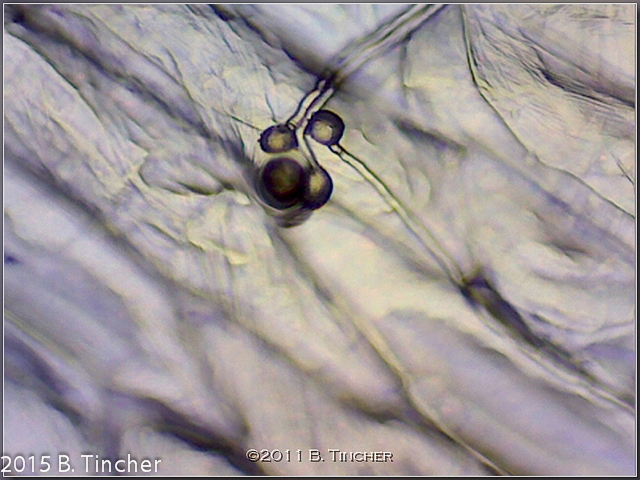
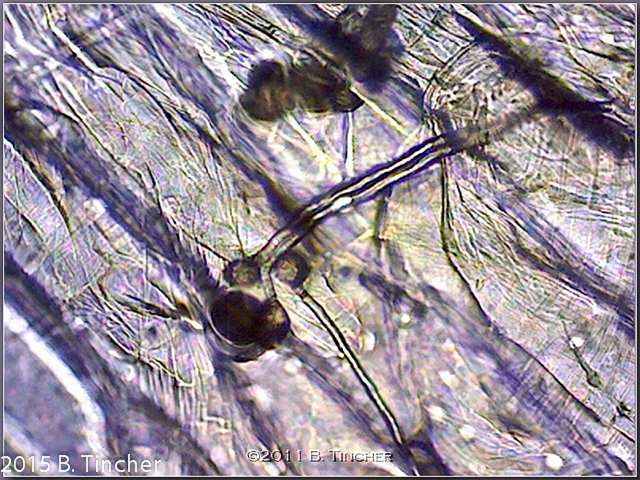

Short youtube video:
Here are killed (negative control) and non killed pollen samples tested using a 0.1% MTT stain solution. Both samples were incubated together.
Pollen had been frozen for 16m in standard residential refrigerator/freezer
(Pollen verified viable by pollen tube growth)
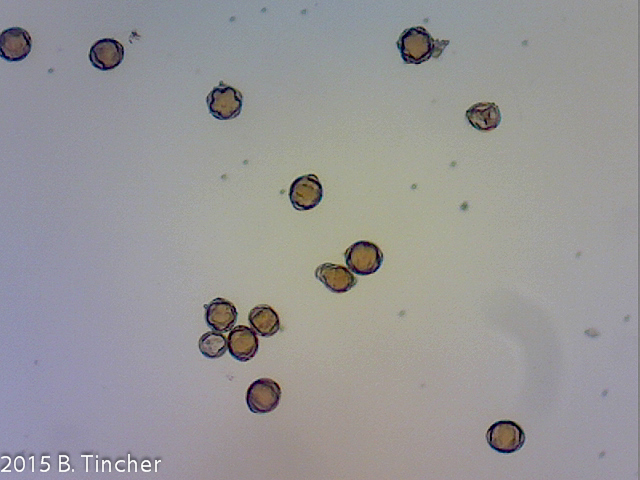
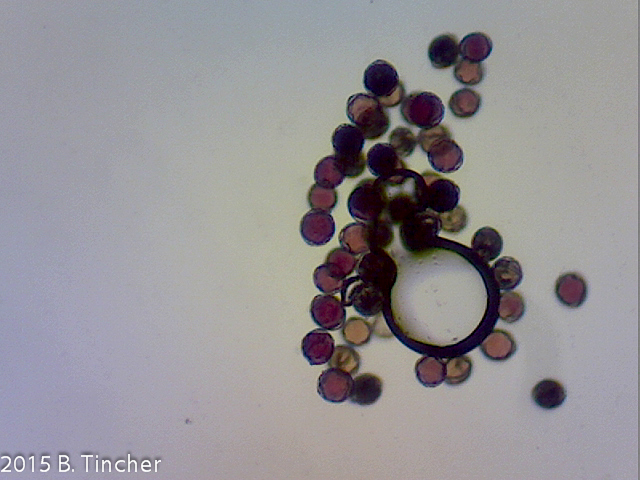
It looks like staining with 0.1% MTT may be a good indicator of pollen viability. I would want to do comparisions using different methods for killing the pollen just to make sure.
Special thanks to Jennifer Lei for providing me with the MTT solution and testing protocol.
